Chroma™
Noxopharm’s science-driven strategy has led to the development of its Chroma™ technology platform. Our highly skilled R&D team has extensive expertise in the development of drug candidates based on a scaffold structure known as functionalised benzopyrans; a structure that is found in a range of medications.
Through methodically creating adaptations based on this scaffold structure, Noxopharm has generated a library of unique drug candidates that share novel bioactive properties to enhance anti-cancer activity. The majority of these valuable early-stage assets in the Chroma platform predominantly signal anti-cancer activity, with several of the drug candidates having the potential to block inflammation as well.
Pancreatic Cancer
A number of molecules are being critically assessed in the Chroma platform. The most advanced is CRO-67, which has demonstrated a unique ‘dual-cell’ activity against pancreatic cancer and the surrounding barrier cells in preclinical studies using cells from patient tumours.
Click on image below to view video

Tumours in the pancreas are surrounded by a dense barrier layer that make them more resistant to both chemotherapy and the body’s natural immune system. The cells in this barrier (known as CAF cells or cancer-associated fibroblasts) also promote the growth and spread of the cancer, meaning that this barrier makes pancreatic cancer particularly aggressive and difficult to treat. The challenge is that any anti-cancer treatment needs to get through the barrier layer and then to the tumour itself.
To address this challenge, UNSW Sydney has recently developed a world-first model of pancreatic cancer for research purposes, using cancer tissue samples surgically removed from pancreatic cancer patients. A recent study by UNSW applied the Noxopharm drug candidate CRO-67 to cancer samples in this model. After 12 days CRO-67 was shown to have dual-cell activity, killing both the cancer cells and the surrounding barrier cells.
This world-first study demonstrates CRO-67 as a novel dual-cell therapy, potently destroying both the tumour and its surrounding barrier. These highly promising results will now drive further studies to maximise the potential of this new approach to pancreatic cancer treatment.
Download the Chroma Pancreatic Cancer pdf for more detail
Glioblastoma
Glioblastoma is the most frequent and lethal type of brain cancer, accounting for two-thirds of all Australian brain cancers. It remains an incurable disease with a median survival time of just 15 months after diagnosis. Today there are only a few treatment options available, and after initial treatment recurrence of the disease is almost inevitable.
The global glioblastoma market was worth around US$2.9 billion in 2022 and is expected to grow at an annual rate of 8.8%.
Noxopharm and the University of South Australia (UniSA) are working on two novel preclinical drugs developed via Noxopharm’s Chroma platform. These drugs have been tested on an extensive biobank of patient-derived tumour explant organoids, a state-of-the-art glioblastoma explant model.
The figure below shows that two novel drugs, known as CRO-70 and CRO-71, significantly reduced the growth of these glioblastoma explants by an average of 75.94% and 75.87% respectively versus the untreated controls. The four asterisks represent a p-value of less than 0.0001, indicating highly statistically significant results.
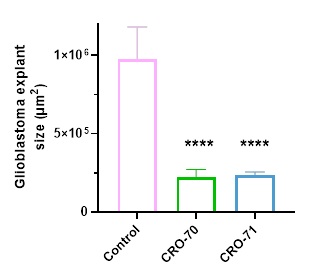
Preliminary analysis of CRO-70 and CRO-71 also demonstrated that these drugs could cross the blood-brain barrier, which is an important protective filter for the brain that most drugs do not manage to cross.
An early animal study has resulted in a favourable safety and toxicity profile for both drugs.
Inflammation
Our team of scientists and collaborators have also discovered that various molecules under development in the Chroma platform are displaying anti-inflammatory properties with potential applications in the treatment of autoimmune disease and hyperinflammatory conditions through the blockade of a target known as TBK1.
TBK1 is a pivotal protein located downstream of multiple inflammatory triggers, which conveys inflammation signals to allow the release of inflammatory factors aimed at clearing infections. However, when excessive inflammation occurs, TBK1 activity can result in significant tissue damage. Noxopharm, along with our collaborator Hudson Institute of Medical Research, is investigating drug candidates that may inhibit TBK1 and could have applications in the treatment of autoimmune diseases such as motor neurone disease, rheumatoid arthritis, multiple sclerosis and type 1 diabetes mellitus.
This excessive inflammation can also be seen in infections like COVID-19, leading to complications, hospitalisations and possibly long COVID. The Victorian government has granted Hudson Institute a $1.45 million grant to investigate this potential application for TBK1 inhibitors. Noxopharm, through its subsidiary Pharmorage, is named on the grant as Hudson Institute’s collaborator to commercialise any promising drug candidates generated by this research.
CRO-67, CRO-70 and CRO-71 are not approved for use in Australia or any other country.


